Valdirene Evans
Global President Texture Solutions and Member of Executive Board
Part of Ingredion Incorporated (NYSE: INGR), a global innovative ingredient solutions provider, we partner with formulators seeking functional materials to enhance formulation performance and overcome their technical challenges.
Our solutions include binders, fillers, superdisintegrants, lubricants, gelatin replacers for softgel, emulsions for encapsulation, viscosifiers, and parenteral APIs.
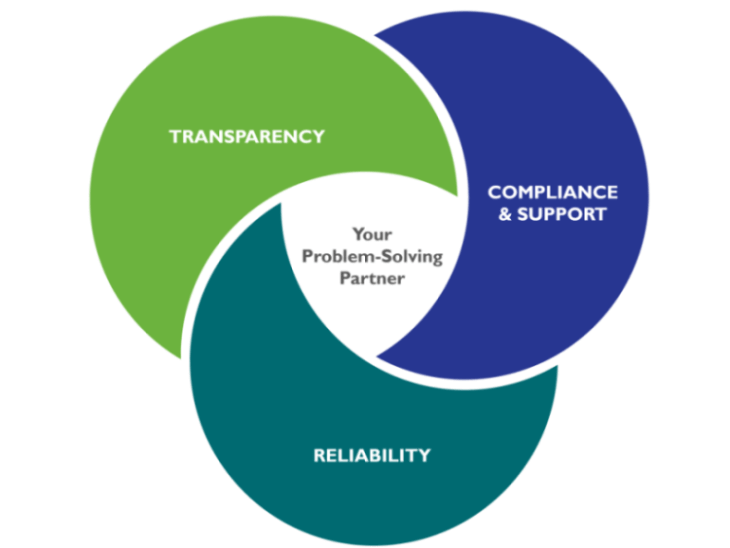
We combine GMP Facilities, Regulatory Assistance, Global presence, and collaborative Technical Support to be Your formulation Problem-Solving Partner.
We are committed on delivering solutions to formulation challenges, with relevant technical
and regulatory support globally.
Transparency
We focus on providing proactive problem-solving.
Compliance & Support
Through a dedicated team, we provide Global Regulatory assistance and Collaborative technical support.
Reliable Supply
We have over 50 years of legacy supplying parenteral grade anhydrous dextrose, high function specialty starches, pharma grade mannitol, and other polyols to pharmaceutical formulators globally. We are now adding other specialty excipients, all manufactured under cGMPs.
Global President Texture Solutions and Member of Executive Board
Business Director
Sales Sr. Manager
Sales Manager
Commercial Director, India, Bangladesh, and Sri Lanka
Sales Manager
Key Account Manager
Key Account Manager
Technical Sales
Sales Manager
Sales Director
Technical Sales Manager
Senior Technical Sales
R&D Manager
Excipient Formulation Scientist
Regulatory Manager
Senior QMS Specialist
Regulatory Affairs
Regulatory Affairs
Regulatory
Director Supply Chain Operations
Global Strategic Planning Manager
Supply Chain Manager
Sampling
Providing cGMP mannufacturing and EIP documentation
We manufacture our excipients using the highest quality raw materials and in adherence to cGMP standards published by IPEC (International Pharmaceutical Excipients Council) in the Good Manufacturing Guide.
We actively participate in IPEC to contribute to the strengthening of standards that assure patient safety and efficacy.
Our plant quality teams monitor our products through rigorous in-process testing and conduct annual product reviews to ensure we are in compliance with and meet USP-NF, Ph. Eur. , JP and IP requirements.
Our regulatory team is staffed with pharmaceutical industry-trained experts. They have built a full range of documents customers require to assist with product registration globally, including in China.
You can find a summary of the regulatory documentation available for each product in the respective EIP Index (Excipient Information Package Index). That contains a list of the documents available. Those marked as Basic in the EIP Index are available to download from the website. The documents marked as On Demand can be accessed via written request to pharma.support@ingredion.com
Our central laboratory in Bridgewater, NJ, USA, is well-equipped with experts in processing and applications to bring a fundamental understanding of structure-function relationships. In 2024, we opened a new applications laboratory in Ahmedabad, India, staffed by experienced formulators.
In addition, our business partners bring their own laboratories to support our customers globally. We seek to provide customers with relevant technical information to enable them to solve their formulation challenges quickly.
Discover how our pharma solutions can
enhance formulation performance and
overcome your technical challenges.